

1 Weigao Road,Torch Hight-Tech Industrial Development Zone, Weihai, Shandong Province, China
 鲁公网安备 37100302000150号
鲁公网安备 37100302000150号

Follow Us


1 Weigao Road,Torch Hight-Tech Industrial Development Zone, Weihai, Shandong Province, China
 鲁公网安备 37100302000150号
鲁公网安备 37100302000150号

Follow Us
The disposable sterile drainage tube (Registration No. : 20242140583) designed and developed by the Medical Products R&D Center of Weigo Shares has recently obtained the registration certificate. This new product can be combined with a negative pressure suction device to effectively solve the problem of surgical smoke generated during endoscopic surgery.
In the modern medical field, with the rapid development of science and technology, more and more high-tech are applied in clinical practice, which greatly improves the level of diagnosis and treatment. Among them, endoscopic surgery is highly respected because of its characteristics of less trauma and quick recovery. However, the problem of surgical smoke caused by electrotome, endoscope and other operations has been troubling the medical staff, affecting the surgical field of vision, but also may bring potential health risks. At present, there is a lack of disposable consumable products on the market to solve this problem, and the usual way is to use a large ventilation system, an electric knife and pen with smoke exhaust function, or rely on medical personnel to wear masks. To this end, we have specially developed a single use sterile drainage tube to solve this long-standing problem.
This product uses a U-shaped tee design, cleverly combined with the negative pressure suction device, can achieve efficient smoke exhaust and drainage functions during the operation, not only to provide medical staff with a clearer surgical field of view, but also to avoid surgical smoke harm to the health of medical staff and patients.
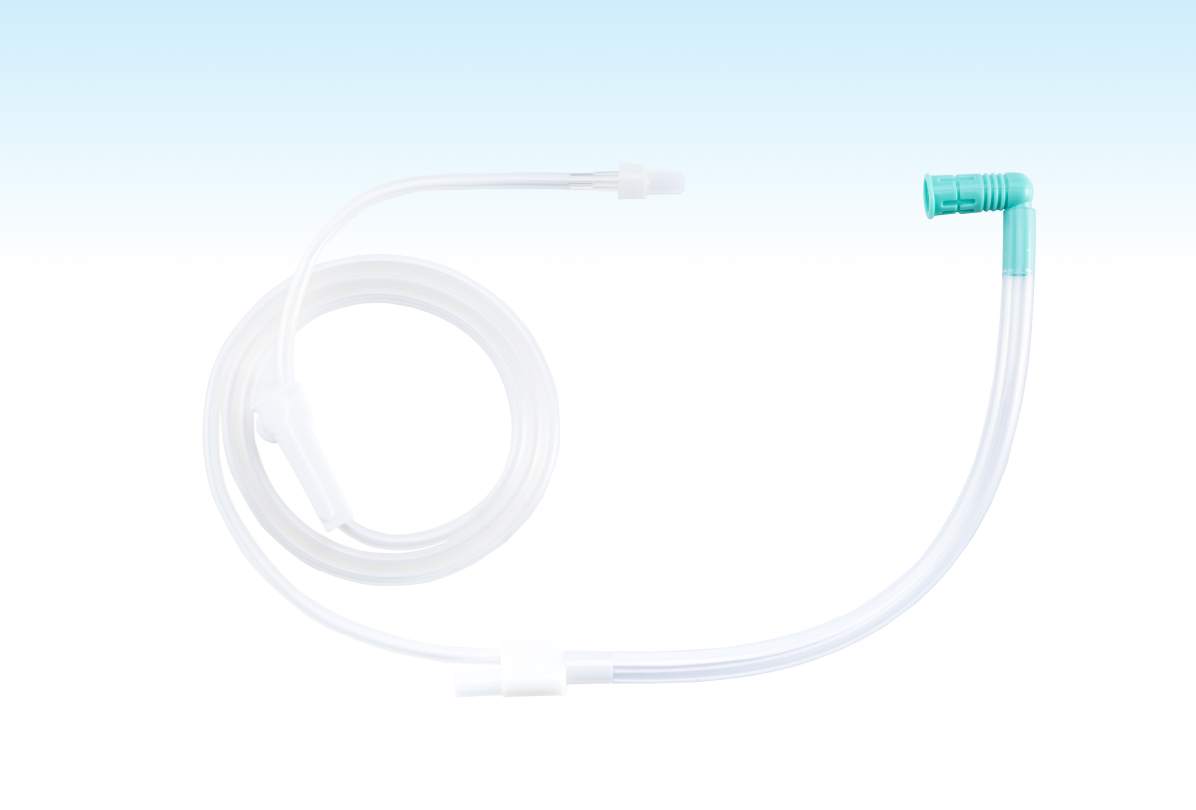
In addition to the basic configuration, the product is currently available in two optional accessories: flow regulator and screw two-way caps. The flow regulator can control the gas flow, and the spiral two-way cap can effectively protect the exhaust pipe joint from contamination. Through the combination of basic configuration and selection, four models A, B, C and D are formed to meet the diversified clinical application needs.