

1 Weigao Road,Torch Hight-Tech Industrial Development Zone, Weihai, Shandong Province, China
 鲁公网安备 37100302000150号
鲁公网安备 37100302000150号

Follow Us


1 Weigao Road,Torch Hight-Tech Industrial Development Zone, Weihai, Shandong Province, China
 鲁公网安备 37100302000150号
鲁公网安备 37100302000150号

Follow Us
The syringe of AK104 (Cusimlimab) developed by Akeso Biopharma has been approved for marketing.
On September 30, 2024, the official website of the National Medical Products Administration (NMPA) of China announced that the marketing application for AK102 (Inclisiran injection), a Class 1 new drug developed by Akeso Biopharma, has been officially approved. The indications are as follows: On the basis of diet control, it is used in combination with statins, or in combination with statins and other lipid-lowering therapies, for adult patients with primary hypercholesterolemia (including heterozygous familial and non-familial hypercholesterolemia) and mixed dyslipidemia who still cannot reach the target level of low-density lipoprotein cholesterol (LDL-C) after receiving medium-dose or higher-dose statin treatment.

The two indications for treating hypercholesterolemia of Inclisiran® (Inclisiran, PCSK9), independently developed by Akeso Biopharma, have been approved, making it the first approved product in the non-tumor field.
Inclisiran injection is an innovative PCSK9 monoclonal antibody independently developed by Akeso Biopharma. It is mainly used for the treatment of primary hypercholesterolemia and mixed hyperlipidemia, including patients with heterozygous familial hypercholesterolemia (HeFH) and those with hypercholesterolemia who also suffer from atherosclerotic cardiovascular diseases. It restores the expression level of low-density lipoprotein receptor (LDL-R) by specifically binding to PCSK9 and blocking its interaction with LDL-R, thereby enhancing the clearance ability of low-density lipoprotein cholesterol (LDL-C) in plasma.
The drug packaging adopted for Inclisiran injection is the overall solution of "prefilled syringe + auto-injector pen" provided by Shandong Weigao Pureasy Pharmaceutical Packaging Co., Ltd.
Activation of the registration number for the Weigao Pureasy auto-injector pen.

The Weigao Pureasy auto-injector pen has activated the Class A certificate through the joint review and approval with the preparation.
What is an auto - injector pen?
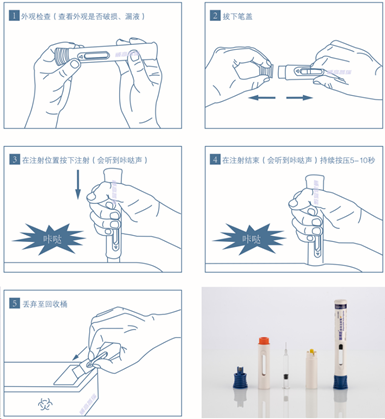
The disposable auto-injector pen is a safe injection device for patients using specified drugs to administer the medication by themselves.
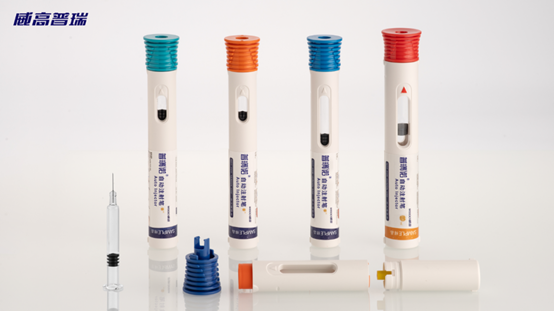
Advantages of the Pureasy Auto-injector Pen:
1. Two-step simple operation.
2. The needle is hidden throughout the process.
3. Auditory and visual feedback during the injection process.
4. Passive needle locking structure.
5. Optimized ergonomic design provides patients with a good operational experience.
6. Smooth cylindrical tube wall, easy to label; large area that can accommodate more information.
Advantages of the customized platform:
1. Initiate and execute customer requirements according to the User Requirements Specification (URS).
2. Visual indicators for guiding correct drug administration, which can be customized according to customer needs.
3. The depth of the needle can be customized to optimize drug absorption.
Usage Instructions:
One-stop solution provider for self-administration devices.
Weigao Pureasy was founded in 2005. It is the first manufacturer of prefilled drug delivery systems in China. Its R & D team has over 30 years of experience in the development of self-administration devices. Based on prefilled products, it continuously meets market demands and develops new products.
The series of injection pen products can manufacture their own accessories, ensuring product compatibility, stable quality and guaranteeing the delivery cycle.
1.Independent research and development, independent manufacturing, and possession of independent patents.
2.Supported by authoritative and professional R & D teams and data verification materials.
3.Accept customizations from customers with special functions and special requirements.
As a leading enterprise in innovative biopharmaceuticals, Akeso Biopharma hopes to develop new drugs that are globally pioneering and represent the best therapies among similar drugs through efficient and breakthrough R & D innovations, aiming to become a world-leading biopharmaceutical enterprise.
As a partner of pharmaceutical enterprises, Weigao Pureasy, with the corporate mission of "Innovating drug delivery systems and experiencing a better life", will continue to uphold high-quality, safe, economical and impeccable services, make new contributions to promoting the development of the pharmaceutical industry and improving people's living standards, and jointly contribute our strength to the development of national enterprises and the pharmaceutical and health fields.
Akeso Biopharma.
Akeso Biopharma was founded by an experienced international team. It focuses on major disease areas such as tumors, autoimmune diseases, inflammations, and metabolic disorders, concentrating on the unmet clinical needs worldwide. It has created a unique end-to-end Akeso all-round new drug research and development platform (ACE Platform), and established a research and development innovation system with the Tetrabody bispecific antibody development technology, antibody-drug conjugate (ADC) technology platform, mRNA technology platform and cell therapy technology as the core, as well as an international-standard GMP production system and a commercialization system with an advanced operation mode.
Akeso Biopharma has always been committed to integrating global advantageous resources through efficient and breakthrough research and development innovations, developing new drugs that are globally pioneering and the best among their peers, providing affordable innovative antibody drug solutions for patients around the world, continuously creating more commercial and social values, and becoming a world-leading biopharmaceutical enterprise.
Weigao Pureasy
Shandong Weigao Pureasy Pharmaceutical Packaging Co., Ltd., as the first manufacturer of prefilled syringes in China to obtain the registration certificate, took the lead in breaking the monopoly of foreign enterprises on prefilled series products in the Chinese market and realized the localization of prefilled packaging materials for vaccines. Its product varieties cover the entire series of prefilled syringe specifications, including prefilled syringes with a volume ranging from 0.5 ml to 50 ml, with or without needles, made of glass or COP materials, prefilled vials, auto-injector pens, pen injectors, injection needles and other full series of products, which comply with the European Pharmacopoeia, the United States Pharmacopoeia and the Japanese Pharmacopoeia. It provides a "one-stop" solution for the prefilled drug delivery system for pharmaceutical enterprises.